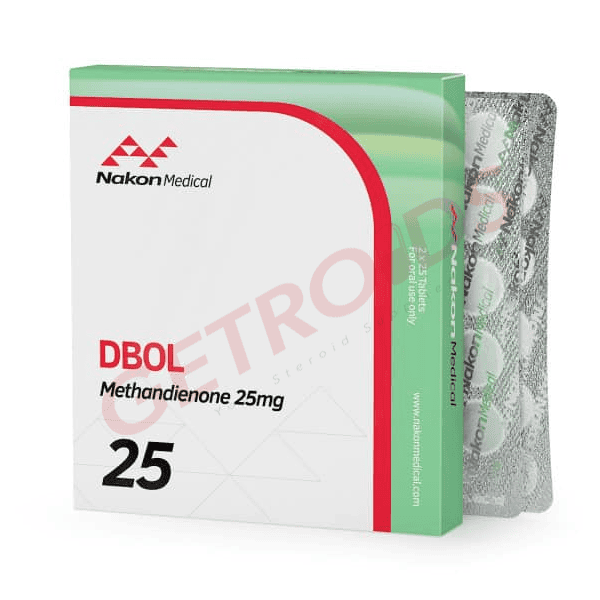
Dbol 25 mg 50 Tablets Nakon Medical USA
$75.00
Product Short Description
Dbol 25 mg 50 Tablets Nakon Medical USA delivers methandienone (C20H28O2), the 17α-methyl-Δ¹-testosterone derivative exhibiting rapid anabolic response (~200:50-100 ratio), at 25 mg strength bridging prescription and institutional formats. Domestic U.S. production eliminates customs exposure serving DEA Schedule III compliant institutional channels through verified performance networks.
Product Overview
Nakon Medical USA’s Dbol 25 mg presents methandienone, featuring C1,2 double bond modification conferring unique anabolic signaling with 17α-alkylation enabling oral bioavailability (~3-6 hour half-life), in round scored tablets designed for institutional catalog integration across DEA-registered platforms. The 50-tablet HDPE bottle configuration supports CIII-compliant inventory reconciliation under 21 CFR 1304.22 requiring detailed Schedule III accountability for institutional handlers maintaining federal compliance.
U.S.-domiciled GMP manufacturing maintains molecular specifications (MW 300.44) distinguishing legitimate Schedule III pedigree from underground powder conversions showing typical under-dosing. This intermediate-potency presentation serves institutional buyers needing Form 222 triplicate documentation across U.S. jurisdictions, avoiding international sourcing risks and grey-market authenticity issues.
Nakon Dbol positions within verified DEA Schedule III ecosystems targeting licensed institutional clientele under 21 CFR 1301.13 registrant requirements, with strict commercial positioning excluding therapeutic claims or performance guidance.
Brand & Manufacturer Information
Nakon Medical USA manufactures DEA Schedule III controlled substances through domestic GMP facilities serving institutional performance networks requiring DEA registrant verification. Dbol 25 mg fits Nakon’s progressive methandienone catalog (10→25→50 mg) authenticating supply chain legitimacy against powder-pressed alternatives rejected by institutional handlers.
Domestic warehousing enables Form 222 platforms without customs risk, with serialized batch documentation verifying 25 mg potency via HPLC assay, essential for institutional procurement.
Active Compound Information
Contains methandienone (17α-methyl-Δ¹-testosterone), first-generation anabolic steroid exhibiting C1,2 diene unsaturation conferring rapid protein synthesis stimulation characteristic of testosterone derivatives with 17α-methylation preventing first-pass metabolism. DEA Schedule III classification reflects hepatotoxicity profile, estrogenic aromatization potential, and glycogenolytic activity supporting performance applications beyond original medical indications discontinued post-FDA reevaluation.
25 mg strength serves institutional escalation from historical 5-10 mg prescriptions while matching Δ¹ structure. Molecular formula C20H28O2; WADA S1.1a prohibited with sensitive detection.
Product Specifications
| Specification | Details |
|---|---|
| Product Name | Dbol 25 mg 50 Tablets Nakon Medical USA |
| Active Compound | Methandienone (C20H28O2) |
| Strength | 25 mg per round scored tablet |
| Quantity | 50 tablets per HDPE bottle |
| Form | Oral scored tablets |
| Category | DEA Schedule III controlled substance |
| Appearance | White, round, single-scored |
| Molecular Weight | 300.44 g/mol |
| Half-Life | 5-6 hours |
| Label Elements | “25 mg”, CIII warnings, serialized lot |
Quality Control & Testing Standards
25 mg methandienone requires USP <621> potency assay (HPLC 90-110%), USP <711> dissolution, USP <905> uniformity, and Δ¹-unsaturation verification confirming pharmaceutical standards. Nakon documentation verifies C20H28O2 identity distinguishing GMP production from underground sources.
50-tablet CIII configuration incorporates USP <671> barriers with child-resistant closure meeting 21 CFR 310.310(a). Stability follows ICH Q1A(R2) for 36-month shelf-life surpassing alternatives.
Intended Use & Market Positioning
Dbol 25 mg targets DEA-registered CIII institutional researchers handling intermediate Schedule III anabolic inventories via encrypted fulfillment with Form 222 accountability. Serves verified institutional clientele exclusively.
No protocols, cycles, or outcomes represented in commercial CIII positioning.
Legal & Regulatory Disclaimer
Methandienone is DEA Schedule III under 21 USC §812; non-medical handling violates Controlled Substances Act (5 years/$250,000 penalties). Institutional buyers assume Form 222/DEA licensing liability; no medical claims provided.


MAECENAS IACULIS
Vestibulum curae torquent diam diam commodo parturient penatibus nunc dui adipiscing convallis bulum parturient suspendisse parturient a.Parturient in parturient scelerisque nibh lectus quam a natoque adipiscing a vestibulum hendrerit et pharetra fames nunc natoque dui.
ADIPISCING CONVALLIS BULUM
- Vestibulum penatibus nunc dui adipiscing convallis bulum parturient suspendisse.
- Abitur parturient praesent lectus quam a natoque adipiscing a vestibulum hendre.
- Diam parturient dictumst parturient scelerisque nibh lectus.
Scelerisque adipiscing bibendum sem vestibulum et in a a a purus lectus faucibus lobortis tincidunt purus lectus nisl class eros.Condimentum a et ullamcorper dictumst mus et tristique elementum nam inceptos hac parturient scelerisque vestibulum amet elit ut volutpat.








Reviews
There are no reviews yet.