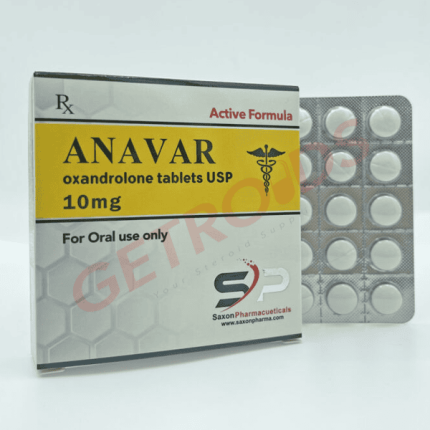
Anavar 10 mg 50 Tablets Nakon Medical USA
$109.00
Product Short Description
Anavar 10 mg 50 Tablets Nakon Medical USA delivers oxandrolone (C19H30O3), the 17β-hydroxy-17α-methyl-2-oxa-5α-androstan-3-one derivative exhibiting mild anabolic potency (~322-630:24 ratio) with minimal androgenicity, at exact 10 mg strength replicating historical Oxandrin formulation. Domestic U.S. production eliminates customs exposure serving DEA Schedule III compliant institutional channels exclusively through verified performance networks.
Product Overview
Nakon Medical USA’s Anavar 10 mg presents oxandrolone, featuring unique 2-oxa lactone ring modification conferring exceptional oral bioavailability (~9-10 hour half-life) and lowest hepatotoxicity among 17α-alkylated steroids, in small oval white scored tablets matching Oxandrin (BTG) morphology for institutional catalog integration across DEA-registered platforms. The 50-tablet HDPE bottle configuration supports CIII-compliant inventory reconciliation under 21 CFR 1304.22 requiring detailed Schedule III tracking for institutional handlers maintaining federal compliance.
U.S.-domiciled GMP manufacturing maintains molecular specifications (MW 306.44) matching FDA historical reference standards while distinguishing legitimate Schedule III pedigree from underground powder conversions exhibiting characteristic under-dosing. This authentic presentation serves institutional buyers requiring verified batch traceability through serialized lot numbering essential for Form 222 triplicate documentation across 50 U.S. jurisdictions, eliminating international sourcing risks inherent to grey-market alternatives.
Nakon Anavar positions exclusively within verified DEA Schedule III ecosystems targeting licensed institutional clientele demonstrating controlled substance handling protocols under 21 CFR 1301.13 registrant requirements.
Brand & Manufacturer Information
Nakon Medical USA manufactures DEA Schedule III controlled substances through domestic GMP facilities serving institutional performance networks requiring rigorous DEA registrant verification prior to encrypted fulfillment. Anavar 10 mg establishes Nakon’s mild anabolic oral catalog alongside Primo/Winstrol maintaining dosage standardization while authenticating supply chain legitimacy versus powder-pressed underground alternatives rejected universally by institutional handlers.
Domestic climate-controlled warehousing supports Form 222 platforms eliminating customs seizure risk while serialized batch documentation verifies 10 mg potency through independent HPLC assay distinguishing Nakon from non-pharmaceutical origins lacking controlled substance pedigree essential for institutional procurement.
Active Compound Information
Contains oxandrolone (17β-hydroxy-17α-methyl-2-oxa-5α-androstan-3-one), DHT-derived anabolic steroid exhibiting 2-oxa lactone ring substitution conferring 6x testosterone anabolic potency with 0.3x androgenic activity characteristic of non-17α-methyl alkylated steroids enabling extended oral administration with minimal liver stress. DEA Schedule III classification reflects cholesterol profile alteration potential and mild HPTA suppression supporting applications beyond discontinued medical indications.
10 mg strength precisely matches historical Oxandrin 10 mg (NDC 54487-454-30) reference while 50-tablet count supports institutional packaging. Molecular formula C19H30O3; WADA S1.1a prohibited globally.
Product Specifications
| Specification | Details |
|---|---|
| Product Name | Anavar 10 mg 50 Tablets Nakon Medical USA |
| Active Compound | Oxandrolone (C19H30O3) |
| Strength | 10 mg per oval scored tablet |
| Quantity | 50 tablets per HDPE bottle |
| Form | Oral scored tablets (Oxandrin equivalent) |
| Category | DEA Schedule III controlled substance |
| Appearance | White, oval, single-scored |
| Molecular Weight | 306.44 g/mol |
| Half-Life | 9-10 hours |
| Label Elements | “10 mg”, CIII warnings, serialized lot |
Quality Control & Testing Standards
10 mg oxandrolone mandates USP <621> potency assay (HPLC 95-105% label claim), USP <711> dissolution (Q=85% 30 minutes), USP <905> uniformity, and lactone ring verification confirming pharmaceutical-grade specifications matching Oxandrin monographs. Nakon batch records document C19H30O3 identity distinguishing legitimate GMP production from underground conversions.
50-tablet CIII configuration incorporates USP <671> HDPE barriers preventing oxidative degradation while child-resistant closure satisfies 21 CFR 310.310(a) Schedule III requirements. Domestic stability maintains ICH Q1A(R2) 40°C/75%RH confirming 36-month shelf-life.
Intended Use & Market Positioning
Anavar 10 mg serves DEA-registered CIII institutional researchers managing mild Schedule III anabolic inventories exclusively through encrypted fulfillment requiring Form 222 accountability. Prescription-equivalent positioning serves verified institutional clientele.
Zero protocols, cycles, stacking, PCT, or outcomes represented maintaining commercial CIII positioning.
Legal & Regulatory Disclaimer
Oxandrolone constitutes DEA Schedule III under 21 USC §812; non-medical handling violates Controlled Substances Act (5 years/$250,000 penalties). Institutional buyers assume Form 222/DEA licensing liability; no medical claims provided.


MAECENAS IACULIS
Vestibulum curae torquent diam diam commodo parturient penatibus nunc dui adipiscing convallis bulum parturient suspendisse parturient a.Parturient in parturient scelerisque nibh lectus quam a natoque adipiscing a vestibulum hendrerit et pharetra fames nunc natoque dui.
ADIPISCING CONVALLIS BULUM
- Vestibulum penatibus nunc dui adipiscing convallis bulum parturient suspendisse.
- Abitur parturient praesent lectus quam a natoque adipiscing a vestibulum hendre.
- Diam parturient dictumst parturient scelerisque nibh lectus.
Scelerisque adipiscing bibendum sem vestibulum et in a a a purus lectus faucibus lobortis tincidunt purus lectus nisl class eros.Condimentum a et ullamcorper dictumst mus et tristique elementum nam inceptos hac parturient scelerisque vestibulum amet elit ut volutpat.









Reviews
There are no reviews yet.