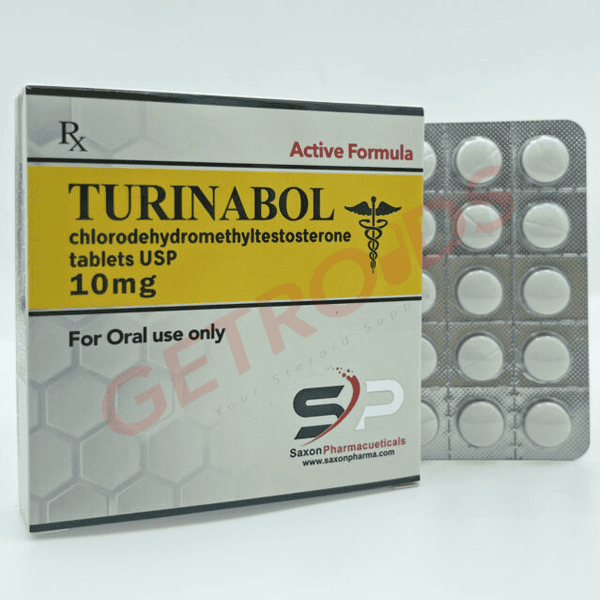
Turinabol 10 mg 50 Tablets Saxon Pharma USA
$95.00
Product Short Description
Turinabol 10 mg 50 Tablets Saxon Pharma USA delivers chlorodehydromethyltestosterone (C20H27ClO2), the 4-chloro-17α-methyltestosterone derivative (~54:6 ratio) exhibiting quality lean gains with zero aromatization, at foundational 10 mg strength. Domestic U.S. production serves DEA Schedule III compliant institutional channels through verified networks.
Product Overview
Saxon Pharma USA’s Turinabol 10 mg presents CDMT, with 4-chloro substitution and Δ1,4-diene structure preventing estrogen conversion while enabling oral bioavailability (~16 hour half-life), in small round scored tablets replicating original Oral-Turinabol form. The 50-tablet HDPE bottle supports CIII inventory reconciliation under 21 CFR 1304.22 for institutional Schedule III handlers.
U.S. GMP manufacturing upholds MW 334.88 specifications distinguishing pedigree from underground variants. Serves buyers needing Form 222 documentation while avoiding grey-market risks.
Saxon positions within DEA Schedule III ecosystems under 21 CFR 1301.13 without therapeutic claims.
Brand & Manufacturer Information
Saxon Pharma USA produces Schedule III substances via domestic GMP serving institutional networks requiring DEA verification. Turinabol 10 mg establishes Saxon’s non-aromatizing oral entry alongside progressive catalog strengths authenticating legitimacy.
Domestic warehousing enables Form 222 platforms with serialized verification of 10 mg potency essential for procurement.
Active Compound Information
Chlorodehydromethyltestosterone (4-chloro-17α-methylandrosta-1,4-dien-3-one) features 4-chloro modification blocking aromatization for dry anabolic effects with moderate hepatotoxicity from 17α-alkylation. DEA Schedule III; WADA S1.1a prohibited with long detectable metabolites.
10 mg aligns with conservative dosing; formula C20H27ClO2.
Product Specifications
| Specification | Details |
|---|---|
| Product Name | Turinabol 10 mg 50 Tablets Saxon Pharma USA |
| Active Compound | Chlorodehydromethyltestosterone (C20H27ClO2) |
| Strength | 10 mg per round scored tablet |
| Quantity | 50 tablets per HDPE bottle |
| Form | Oral scored tablets |
| Category | DEA Schedule III controlled substance |
| Appearance | White, round, single-scored |
| Molecular Weight | 334.88 g/mol |
| Half-Life | ~16 hours |
| Label Elements | “10 mg”, CIII warnings, lot code |
Quality Control & Testing Standards
10 mg CDMT mandates USP <621> HPLC assay (90-110%), dissolution, uniformity, and chloro-verification. Saxon confirms C20H27ClO2 identity via GMP distinguishing from fakes.
CIII packaging satisfies USP <671> and ICH Q1A(R2) stability.
Intended Use & Market Positioning
Targets DEA-registered CIII researchers via Form 222 fulfillment for institutional inventories.
No protocols represented in CIII positioning.
Legal & Regulatory Disclaimer
CDMT constitutes DEA Schedule III under 21 USC §812; non-medical handling violates CSA (5 years/$250,000 penalties). Buyers assume DEA liability; no claims provided.


MAECENAS IACULIS
Vestibulum curae torquent diam diam commodo parturient penatibus nunc dui adipiscing convallis bulum parturient suspendisse parturient a.Parturient in parturient scelerisque nibh lectus quam a natoque adipiscing a vestibulum hendrerit et pharetra fames nunc natoque dui.
ADIPISCING CONVALLIS BULUM
- Vestibulum penatibus nunc dui adipiscing convallis bulum parturient suspendisse.
- Abitur parturient praesent lectus quam a natoque adipiscing a vestibulum hendre.
- Diam parturient dictumst parturient scelerisque nibh lectus.
Scelerisque adipiscing bibendum sem vestibulum et in a a a purus lectus faucibus lobortis tincidunt purus lectus nisl class eros.Condimentum a et ullamcorper dictumst mus et tristique elementum nam inceptos hac parturient scelerisque vestibulum amet elit ut volutpat.









Reviews
There are no reviews yet.