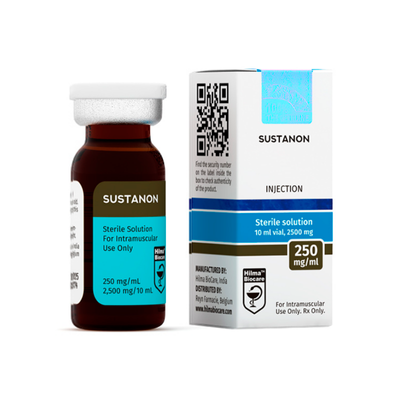
Sustanon 250 Hilma Biocare USA
$95.00
Product Short Description
Sustanon 250 from Hilma Biocare USA delivers 250mg/ml quadruple testosterone ester blend (30mg propionate, 60mg phenylpropionate, 60mg isocaproate, 100mg decanoate) in 10ml multi-dose vial, presented as sterile injectable oil solution for eligible adult consumers. Rubber-stoppered glass vial incorporates flip-top security with color-coded labeling meeting standard concentration verification standards. Positioned for buyers evaluating foundational multi-ester formulations through USA distribution infrastructure.
Product Overview
Sustanon 250 by Hilma Biocare USA standardizes 250mg/ml quadruple testosterone ester blend concentration within 10ml amber glass vial engineered for continental USA performance compound fulfillment. Multi-ester configuration provides sustained testosterone release spanning 15-day average half-life optimized for weekly administration protocols. Hilma Biocare channels this classic presentation toward procurement networks prioritizing stable blood levels within domestic pharmaceutical logistics frameworks.
Brand & Manufacturer Information
Hilma Biocare configures production capacity around multi-ester testosterone injectables maintaining USA operational infrastructure optimized for domestic warehouse-to-warehouse transit velocity and comprehensive batch traceability deployment. Manufacturing standardization emphasizes quantitative matrix consistency across 250mg/ml thresholds while integrating proprietary verification symbology. Wholesale partnerships leverage ester synergy metrics alongside perpetual replenishment cadence reliability critical for institutional distribution sustainability.
Active Compound Information
Sustanon 250 systematically integrates Testosterone Propionate (30mg/ml) providing rapid onset, Testosterone Phenylpropionate (60mg/ml) delivering mid-duration release, Testosterone Isocaproate (60mg/ml) extending intermediate action, alongside Testosterone Decanoate (100mg/ml) conferring long-term sustained delivery—all manifesting characteristic androgenic/anabolic profiles. Quad-ester architecture delivers continuous testosterone coverage combining nitrogen retention, protein synthesis, strength progression through weekly injection maintaining stable plasma concentrations.
Product Specifications
Injectable solution furnishes 250mg/ml total testosterone esters across 10ml multi-dose capacity utilizing pharmaceutical-grade carrier oil matrix with benzyl alcohol preservation. Sterile filtration deployment through 0.22μ pre-filled rubber-stoppered vial incorporating aluminum seal crimp and flip-top security mechanism. Vial documentation enumerates ester ratios (30-60-60-100mg/ml), aggregate volume quantification, sequential lot designation, fabrication temporal reference, and projected pharmacotherapeutic viability timeline.
Quality Control & Testing Standards
Hilma Biocare institutes quantitative chromatographic profiling protocols establishing quadruple-ester concentration conformance alongside pharmacopeial sterility assurance profiling, endotoxin quantification characterization below 0.5 EU/ml, and preservative system efficacy analysis per established monographs. Precursor qualification adjudication precedes esterification via third-party certification protocols while terminal injectable integrity validation incorporates randomized composite sampling against parametric conformance envelopes. Serialization-linked analytical certification dossiers facilitate recipient-independent authenticity adjudication infrastructure.
Intended Use & Market Positioning
Hilma Biocare directs Sustanon 250mg/ml 10ml presentation toward beginner-intermediate clientele executing bulking protocols requiring comprehensive testosterone support through 250-750mg weekly injections over 10-16 week cycles. Multi-ester configuration enables single weekly injection combining immediate/sustained physiological augmentation suitable for TRT replacement or performance enhancement. USA geolocational optimization confers dispatch velocity precedence serving credential-verified recipient cohorts.
Packaging, Storage & Handling
Primary amber glass vial consolidation within protective secondary enclosure integrates dosage authentication cartography, rubber septum penetration verification, flip-top intrusion detection engineering, and microenvironmental sequestration specifications. Preservation ordinance prescribes thermal confinement 15-25°C within inverted orientation repositories mitigating sedimentation layering and oxidative degradation cascades. Protocol mandates crimp seal continuity validation, flip-top functionality confirmation preceding restricted-access conservation domain assignment.
Purchasing & Availability Information
Sustanon 250mg/ml 10ml by Hilma Biocare USA sustains perpetual standing inventory across credentialed electronic transaction architectures with redundant domestic warehouse positioning. Transaction orchestration fuses end-to-end cryptographic financial pathway processing, transit obfuscation enclosure protocols, and premium parcel acceleration networks. Institutional acquisition contingents unlock progressive volumetric economic calibration while discrete transactions receive expedited staging predicated upon eligibility parameter verification.
Legal & Regulatory Disclaimer
Acquisition principals assume unqualified fiduciary accountability for statutory conformity spanning procurement entitlement, possession legitimacy, cross-jurisdictional conveyance authorization, and application permissions pursuant to controlling federal, state, municipal ordinance hierarchies. Hilma Biocare commercializes Sustanon 250 exclusively as parametrically-declared bulk active pharmaceutical matrix; no attestations encompass therapeutic protocol substitution, diagnostic adjunct deployment, prophylactic strategy implementation, or physiological trajectory forecasting. Documentation framework constitutes commercial-informational reference exclusively, expressly prohibiting interpretive counsel substitution.


MAECENAS IACULIS
Vestibulum curae torquent diam diam commodo parturient penatibus nunc dui adipiscing convallis bulum parturient suspendisse parturient a.Parturient in parturient scelerisque nibh lectus quam a natoque adipiscing a vestibulum hendrerit et pharetra fames nunc natoque dui.
ADIPISCING CONVALLIS BULUM
- Vestibulum penatibus nunc dui adipiscing convallis bulum parturient suspendisse.
- Abitur parturient praesent lectus quam a natoque adipiscing a vestibulum hendre.
- Diam parturient dictumst parturient scelerisque nibh lectus.
Scelerisque adipiscing bibendum sem vestibulum et in a a a purus lectus faucibus lobortis tincidunt purus lectus nisl class eros.Condimentum a et ullamcorper dictumst mus et tristique elementum nam inceptos hac parturient scelerisque vestibulum amet elit ut volutpat.







Reviews
There are no reviews yet.