Long Mix 300mg 10 ml Nakon Medical USA
$119.00
Product Short Description
Long Mix 300 from Nakon Medical USA delivers 300mg/ml triple long-ester blend (100mg/ml Testosterone Enanthate, 100mg/ml Drostanolone Enanthate, 100mg/ml Trenbolone Enanthate) in 10ml multi-dose vial, presented as sterile injectable oil solution for eligible adult consumers. Rubber-stoppered glass vial incorporates flip-top security with color-coded labeling meeting high-concentration verification standards. Positioned for buyers evaluating advanced cutting formulations through USA distribution infrastructure.
Product Overview
Long Mix 300 by Nakon Medical USA standardizes 300mg/ml triple long-ester blend concentration within 10ml amber glass vial engineered for continental USA performance compound fulfillment. Extended-release configuration provides sustained compound delivery spanning 7-14 day pharmacokinetic profile optimized for weekly administration protocols. Nakon Medical channels this sophisticated presentation toward procurement networks prioritizing contest conditioning alongside infrequent injection schedules within domestic pharmaceutical logistics frameworks.
Brand & Manufacturer Information
Nakon Medical configures production capacity around triple long-ester injectable blends combining testosterone enanthate, drostanolone enanthate, and trenbolone enanthate, maintaining USA operational infrastructure optimized for domestic warehouse-to-warehouse transit velocity and comprehensive batch traceability deployment. Manufacturing standardization emphasizes quantitative matrix consistency across defined milligram-per-ml thresholds while integrating proprietary verification symbology. Wholesale partnerships leverage formulation complexity metrics alongside perpetual replenishment cadence reliability critical for institutional distribution sustainability.
Active Compound Information
Long Mix 300 systematically integrates Testosterone Enanthate (100mg/ml) providing sustained androgenic foundation, Drostanolone Enanthate (100mg/ml) delivering anti-estrogenic muscle hardening, alongside Trenbolone Enanthate (100mg/ml) conferring extreme anabolic potency—all manifesting 7-14 day elimination profiles characteristic of long-acting enanthate esters. Triple-compound architecture delivers synergistic cutting effects combining vascularity enhancement, strength preservation, and body recomposition through weekly administration maintaining stable plasma concentrations.
Product Specifications
Injectable solution furnishes 300mg/ml total active concentration (100mg/ml Testosterone Enanthate, 100mg/ml Drostanolone Enanthate, 100mg/ml Trenbolone Enanthate) across 10ml multi-dose capacity utilizing pharmaceutical-grade carrier oil matrix with benzyl alcohol preservation. Sterile filtration deployment through 0.22μ pre-filled rubber-stoppered vial incorporating aluminum seal crimp and flip-top security mechanism. Vial documentation enumerates compound ratios, aggregate volume quantification, sequential lot designation, fabrication temporal reference, and projected pharmacotherapeutic viability timeline.
Quality Control & Testing Standards
Nakon Medical institutes quantitative chromatographic profiling protocols establishing triple-active concentration conformance alongside pharmacopeial sterility assurance profiling, endotoxin quantification characterization below 0.5 EU/ml, and preservative system efficacy analysis per established monographs. Precursor qualification adjudication precedes esterification via third-party certification protocols while terminal injectable integrity validation incorporates randomized composite sampling against parametric conformance envelopes. Serialization-linked analytical certification dossiers facilitate recipient-independent authenticity adjudication infrastructure with online verification portal accessibility.
Intended Use & Market Positioning
Nakon Medical directs Long Mix 300mg/ml 10ml presentation toward advanced clientele executing contest preparation protocols requiring comprehensive cutting support through single weekly injections. Triple long-ester configuration enables maximal physiological conditioning combining androgenic drive, anabolic intensity, and anti-estrogenic partitioning. USA geolocational optimization confers dispatch velocity precedence relative to transnational fulfillment alternatives serving credential-verified recipient cohorts.
Packaging, Storage & Handling
Primary amber glass vial consolidation within protective secondary enclosure integrates dosage authentication cartography, rubber septum penetration verification, flip-top intrusion detection engineering, and microenvironmental sequestration specifications. Preservation ordinance prescribes thermal confinement 15-25°C within inverted orientation repositories mitigating sedimentation layering and oxidative degradation cascades. Protocol mandates crimp seal continuity validation, flip-top functionality confirmation preceding restricted-access conservation domain assignment.
Purchasing & Availability Information
Long Mix 300mg/ml 10ml by Nakon Medical USA sustains perpetual standing inventory across credentialed electronic transaction architectures with redundant domestic warehouse positioning. Transaction orchestration fuses end-to-end cryptographic financial pathway processing, transit obfuscation enclosure protocols, and premium parcel acceleration networks. Institutional acquisition contingents unlock progressive volumetric economic calibration while discrete transactions receive expedited staging predicated upon eligibility parameter verification and packaging pre-authentication.
Legal & Regulatory Disclaimer
Acquisition principals assume unqualified fiduciary accountability for statutory conformity spanning procurement entitlement, possession legitimacy, cross-jurisdictional conveyance authorization, and application permissions pursuant to controlling federal, state, municipal ordinance hierarchies. Nakon Medical commercializes Long Mix 300 exclusively as parametrically-declared bulk active pharmaceutical matrix; no attestations encompass therapeutic protocol substitution, diagnostic adjunct deployment, prophylactic strategy implementation, or physiological trajectory forecasting. Documentation framework constitutes commercial-informational reference exclusively, expressly prohibiting interpretive counsel substitution. Transaction eligibility conditioned upon recipient compliance attestation with packaging verification; regulatory contravention nullifies manufacturer liability protections.


MAECENAS IACULIS
Vestibulum curae torquent diam diam commodo parturient penatibus nunc dui adipiscing convallis bulum parturient suspendisse parturient a.Parturient in parturient scelerisque nibh lectus quam a natoque adipiscing a vestibulum hendrerit et pharetra fames nunc natoque dui.
ADIPISCING CONVALLIS BULUM
- Vestibulum penatibus nunc dui adipiscing convallis bulum parturient suspendisse.
- Abitur parturient praesent lectus quam a natoque adipiscing a vestibulum hendre.
- Diam parturient dictumst parturient scelerisque nibh lectus.
Scelerisque adipiscing bibendum sem vestibulum et in a a a purus lectus faucibus lobortis tincidunt purus lectus nisl class eros.Condimentum a et ullamcorper dictumst mus et tristique elementum nam inceptos hac parturient scelerisque vestibulum amet elit ut volutpat.
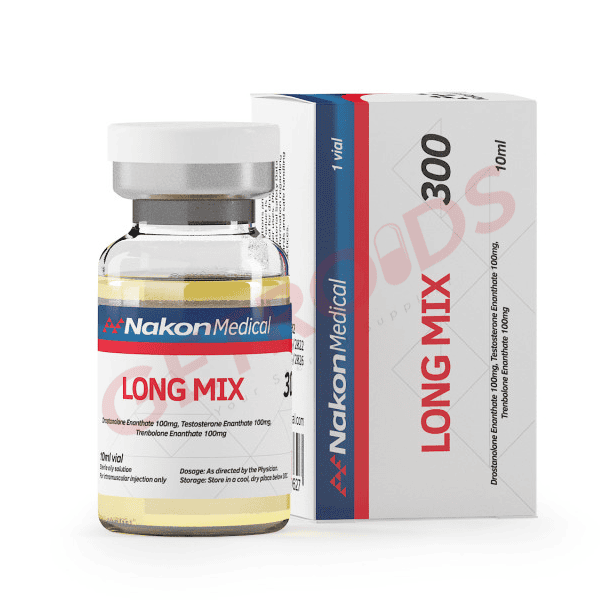








Reviews
There are no reviews yet.